X chromosome inactivation is a fascinating biological process that plays a critical role in cellular function, particularly in females who possess two X chromosomes. Unlike males, who have a single X chromosome, females must silence one of their X chromosomes to achieve genetic balance and prevent overexpression of X-linked genes. This chromosomal silencing mechanism is vital for understanding genetic therapies targeting X-linked diseases, including Fragile X Syndrome and Rett Syndrome. Recent breakthroughs by researchers, including Jeannie T. Lee and her team, highlight how manipulating X chromosome inactivation could unlock new treatment pathways and create effective therapeutic strategies for these conditions. As the scientific community continues to unravel the complexities of X-inactivation, the potential for advancing treatments in X-linked genetic disorders grows, promising hope for countless individuals affected by these conditions.
Exploring the concept of X chromosome inactivation, or the process by which one of the two X chromosomes in females is rendered inactive, reveals a crucial biological adaptation. This phenomenon, also known as chromosomal silencing, ensures that females do not express twice the genetic information found on the X chromosome compared to males. Research on this topic has significant implications for genetic therapies aimed at treating X-linked diseases such as Fragile X Syndrome and Rett Syndrome. By understanding the intricate mechanics of this silencing process, scientists aim to develop innovative treatments that could reactivate certain genes, providing new hope for those affected. As we delve deeper into the realm of X-linked genetic disorders, illuminating strategies to address these challenges becomes increasingly essential.
Understanding X Chromosome Inactivation
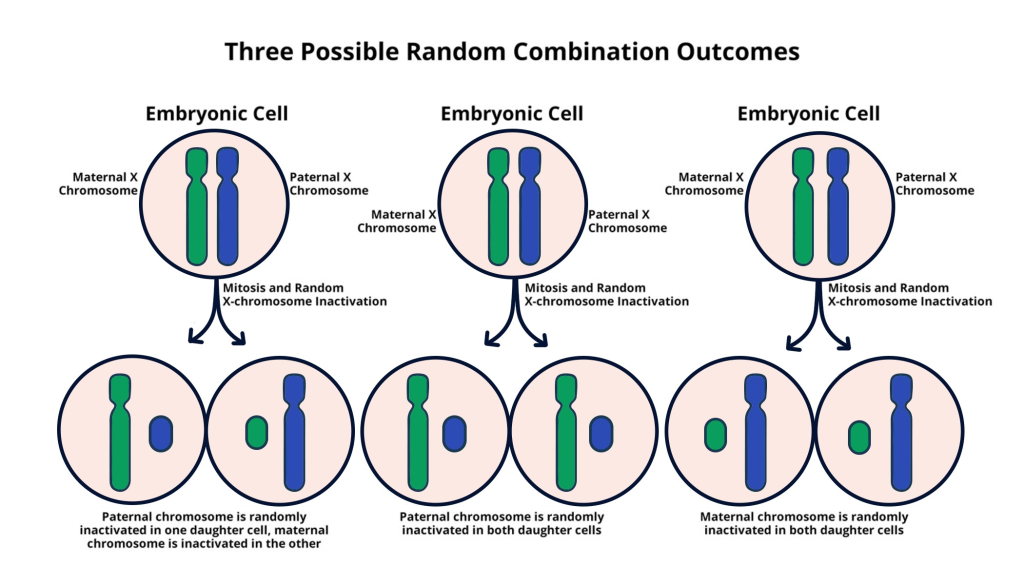
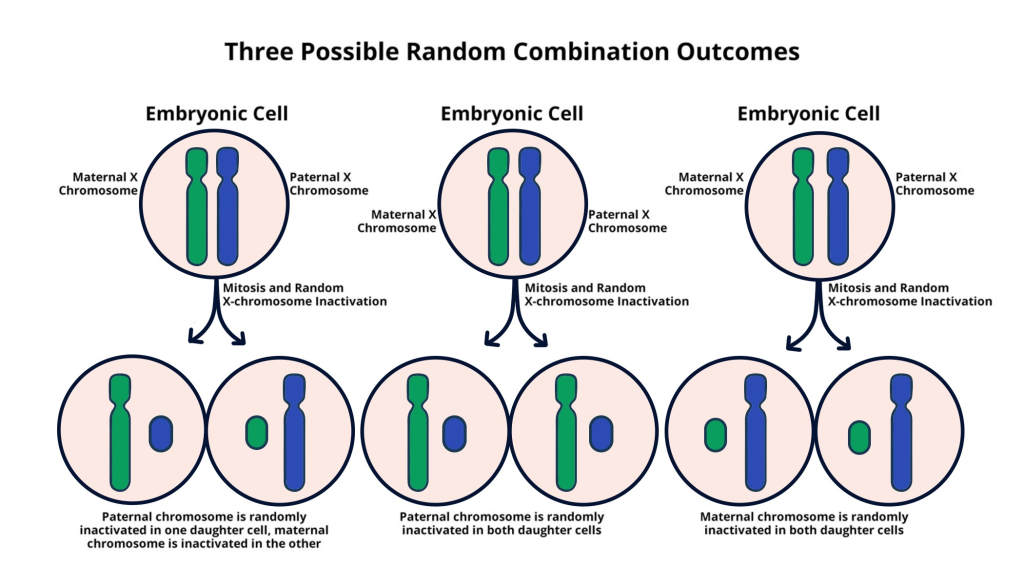
X chromosome inactivation (XCI) is a crucial biological process that ensures dosage compensation for genes located on the X chromosome, particularly in females who have two copies. This mechanism not only silences one of the two X chromosomes in each female cell but also plays a vital role in the regulation of several X-linked diseases, such as Fragile X Syndrome and Rett Syndrome. The inactivation is facilitated by the production of an RNA molecule, called Xist, which modifies the chromosomal environment, effectively silencing the gene it embodies. Researchers, including those led by Jeannie T. Lee, have made significant strides in understanding the complexities of this process, uncovering new insights into how chromosomal silencing mechanisms operate at a cellular level.
By revealing the intricate choreography that dictates XCI, we open doors to potential genetic therapies that could target these silencing mechanisms. For instance, if we can manipulate the process of inactivation, it may be possible to reactivate beneficial genes that are otherwise silenced due to mutations. The implications for therapeutic strategies are enormous, particularly in alleviating the challenges posed by X-linked diseases. As research develops, the hope is that treatments derived from these findings could lead to effective interventions for conditions like Fragile X Syndrome and Rett Syndrome, drastically improving the quality of life for affected individuals.
Frequently Asked Questions
What is X chromosome inactivation and why is it important in genetic therapies?
X chromosome inactivation (XCI) is a process unique to females where one of the two X chromosomes is silenced to ensure that gene dosage remains balanced with that of males, who have only one X chromosome. Understanding XCI is critical in genetic therapies for X-linked diseases, like Fragile X Syndrome, as it holds the potential to unlock therapies that could reactivate the inactivated X chromosome, providing access to healthy gene copies.
How does X chromosome inactivation relate to Fragile X Syndrome treatments?
X chromosome inactivation is particularly relevant to Fragile X Syndrome treatments because the disorder is often caused by mutations on the X chromosome. By developing strategies to unsilence the inactivated X chromosome, researchers are aiming to restore expression of the healthy FMR1 gene, which could alleviate the symptoms associated with Fragile X Syndrome.
What role does chromosomal silencing play in Rett Syndrome research?
In Rett Syndrome research, chromosomal silencing is a key focus because the disorder is linked to mutations on the X chromosome, specifically in the MECP2 gene. By better understanding the mechanisms of X chromosome inactivation, researchers can create potential therapies to activate the silenced copy of the MECP2 gene, offering hope for treating this neurodevelopmental disorder.
Are there any new findings related to X chromosome inactivation that could impact treatments for X-linked diseases?
Recent findings from Jeannie T. Lee’s research reveal novel insights into the processes of X chromosome inactivation, highlighting the role of the Xist RNA molecule in chromosomal silencing. These breakthroughs may pave the way for innovative genetic therapies aimed at reversing X chromosome inactivation, thereby providing new treatment avenues for conditions like Fragile X Syndrome and Rett Syndrome.
Can X chromosome inactivation mechanisms be utilized for gene therapy in X-linked diseases?
Yes, the mechanisms of X chromosome inactivation can be harnessed for gene therapy in X-linked diseases. By developing approaches that target the silencing of the inactive X chromosome, researchers aim to reactivate healthy gene expressions, providing potential treatments for disorders linked to mutations on the X chromosome.
What is the significance of the Xist gene in the context of X chromosome inactivation?
The Xist gene plays a crucial role in X chromosome inactivation by producing an RNA molecule that coats the X chromosome, initiating a series of changes that lead to its silencing. Understanding the function of Xist is essential for potential therapies aimed at reversing X chromosome inactivation, especially in X-linked diseases like Fragile X Syndrome and Rett Syndrome.
How does chromosomal Jell-O relate to the study of X chromosome inactivation?
The term ‘chromosomal Jell-O’ metaphorically describes the composition of the nuclear environment that surrounds chromosomes, including the X chromosome. This gelatinous substance is instrumental in supporting the process of X chromosome inactivation by providing a flexible medium through which the Xist RNA molecule can act, facilitating chromosomal silencing.
What future directions are being explored for genetic therapies targeting X-linked diseases?
Future directions for genetic therapies targeting X-linked diseases include optimizing techniques to unsilence the inactivated X chromosome, conducting safety studies, and moving towards clinical trials. Research is focusing on translating the knowledge of X chromosome inactivation mechanisms into effective treatments for conditions like Fragile X Syndrome and Rett Syndrome.
What challenges remain in understanding X chromosome inactivation?
Despite significant progress, challenges in understanding X chromosome inactivation include elucidating the precise mechanisms by which inactivation occurs and why certain genes remain unaffected after unsilencing. Further research is necessary to unravel these complexities and enhance the development of targeted therapies for X-linked genetic disorders.
| Key Aspect | Details |
|---|---|
| X Chromosome Inactivation | Females have two X chromosomes while males have one, necessitating the inactivation of one copy in females. |
| Research Significance | Decades of research have provided insights into how cells manage X chromosome inactivation, crucial for understanding genetic diseases. |
| Role of Xist | A gene on the X chromosome produces Xist RNA, which modifies the surrounding chromosomal structure, facilitating inactivation. |
| Therapeutic Potential | Emerging therapies could unsilence inactivated X chromosomes, potentially treating conditions like Fragile X and Rett syndromes. |
| Future Directions | Ongoing optimization and safety studies are proposed, leading to clinical trials aimed at validating these treatments. |
Summary
X chromosome inactivation is a fundamental biological process essential for balancing gene expression in females who possess two X chromosomes. This intricate mechanism, studied extensively by Jeannie T. Lee and her team, presents exciting opportunities for therapeutic advancements, particularly for treating genetic disorders related to mutations on the X chromosome, such as Fragile X Syndrome and Rett Syndrome. With new methods under development to potentially reactivate silent genes, the research heralds a promising future in genetic therapy and offers hope to many affected individuals.