X chromosome inactivation is a fascinating biological process that ensures females, who possess two X chromosomes, do not express double the amount of genes encoded on this chromosome compared to males. This phenomenon is critical for understanding genetic disorders such as Fragile X Syndrome and Rett Syndrome, both of which are linked to mutations on the X chromosome. Research led by Jeannie T. Lee at Harvard Medical School has revealed the intricate mechanisms behind this inactivation, providing insight that could pave the way for innovative gene therapy solutions. By manipulating the silencing of the X chromosome, scientists aim to unlock the potential for treating these X-linked genetic disorders, benefitting countless individuals affected by such conditions. The ongoing chromosomal research promises to deepen our understanding of complex genetic interactions and improve therapeutic outcomes for inherited diseases.
The process of X chromosome silencing, also known as Lyonization, represents a key aspect of female genetic regulation, contrasting sharply with the genetic landscape of males who possess a single X chromosome. This essential mechanism plays a significant role in disorders such as Fragile X and Rett syndromes, highlighting the importance of understanding chromosomal behavior in relation to gene expression. As researchers navigate the depths of these genetic phenomena, the intersection of chromosomal research and advanced gene therapy strategies emerges as a beacon of hope for tackling substantial genetic disorders. By demystifying the complexities surrounding X inactivation, we unlock pathways that could lead to groundbreaking treatments, amplifying our ability to combat these challenging conditions. In this light, the exploration of X chromosome inactivation not only advances scientific knowledge but could also transform the landscape of therapeutic possibilities.
Understanding X Chromosome Inactivation
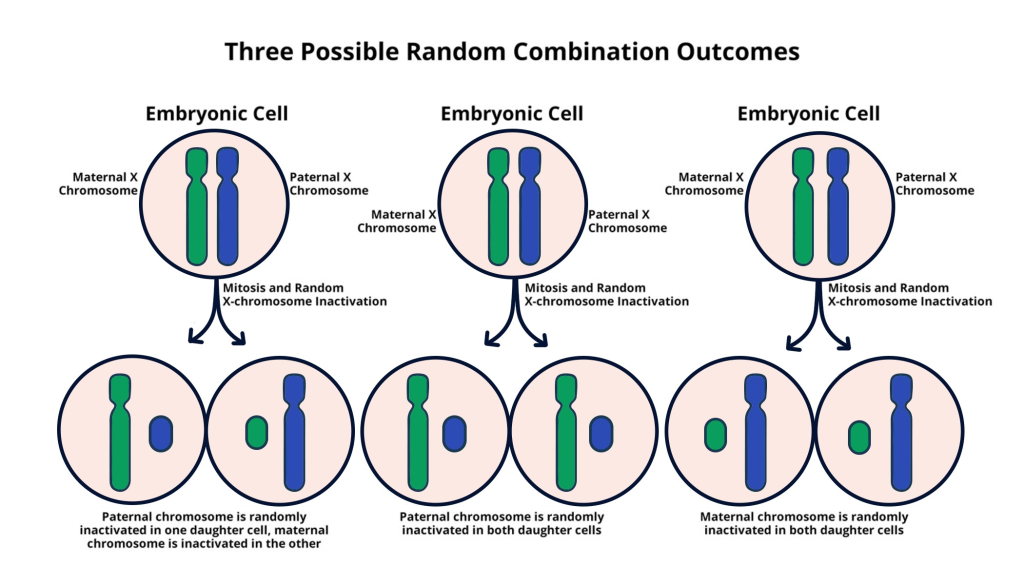
X chromosome inactivation (XCI) is a fascinating biological process that plays a crucial role in genetic expression in females. In mammals, females possess two X chromosomes, and to ensure that they do not produce twice the amount of gene products coded by these chromosomes, one X chromosome is randomly inactivated during early embryonic development. This mechanism not only balances gene expression between sexes but also highlights the complexity of chromosomal interactions within the cell. Researchers, including Jeannie T. Lee and her team, have focused extensively on unraveling the molecular intricacies of XCI, revealing how factors like the RNA molecule Xist interact with the chromosomal architecture to trigger silencing of one X chromosome, ensuring cellular stability and functionality.
Furthermore, the way XCI occurs illustrates the remarkable adaptability of biological systems. The process is not merely a switch being turned off; it involves significant structural alterations dictated by the biochemical environment of the chromosome. Lee’s research has indicated that the ‘Jell-O-like’ substance surrounding chromosomes might play a key role in facilitating this inactivation. By manipulating this process, scientists hope to unlock pathways that could lead to treatments for various genetic disorders associated with mutations on the X chromosome.
In the context of disease, understanding XCI provides insights into conditions such as Fragile X syndrome and Rett syndrome, which are linked to genes located on the X chromosome. Fragile X syndrome, characterized by intellectual disabilities, arises from mutations in the FMR1 gene, whereas Rett syndrome, predominantly affecting females, results from mutations in the MECP2 gene. The research surrounding XCI has demonstrated that by selectively unsilencing the inactivated X chromosome, it is possible to restore normal gene function, presenting a potential therapeutic avenue. This opens up exciting possibilities for gene therapy strategies aimed at correcting the underlying causes of these disorders, which could improve the lives of many individuals affected by such genetic conditions.
The Role of Jell-O-Like Substances in Chromosome Function
The analogy of Jell-O offers a vivid illustration of the cellular environment in which chromosomes operate. The gelatinous substances in question act as a protective and organizational medium for chromosomes, preventing them from entangling and ensuring proper segregation during cell division. This ‘Jell-O’ not only maintains chromosomal integrity but also dynamically influences how genes are expressed, specifically in the context of X chromosome inactivation. The interplay between Xist RNA and the Jell-O-like substance demonstrates a sophisticated mechanism of gene regulation that is essential for normal development and cellular function. As researchers like Jeannie T. Lee explore these interactions, they reveal the importance of these biophysical properties in chromosomal behavior, which could potentially lead to breakthroughs in understanding various genetic disorders.
Beyond facilitating XCI, the implications of this gelatinous environment extend to the realm of other chromosomal research. The biophysical properties of the Jell-O can affect the accessibility of certain gene regions, influencing whether or not they are expressed. Enhanced flexibility, as induced by Xist, allows regulatory molecules to infiltrate the chromosome more effectively. This could have far-reaching consequences not only for X-linked disorders but for a broader range of genetic conditions by elucidating how gene therapy could be fine-tuned for more effective interventions aimed at silencing or activating problematic genes.
Gene Therapy Advances for Fragile X and Rett Syndromes
Gene therapy represents a promising frontier in the treatment of genetic disorders, particularly those linked to X chromosome anomalies such as Fragile X syndrome and Rett syndrome. These disorders, stemming from mutations on the X chromosome, highlight a crucial aspect of gene therapy – the ability to ‘unsilence’ inactivated genes to restore their function. The advances made by Jeannie T. Lee’s team in manipulating X chromosome biophysics indicate that targeted therapies could be developed to reactivate healthy gene expression from the inactivated X chromosome, effectively addressing the root cause of these conditions. As research progresses, the potential for clinical trials will increasingly be an avenue for providing hope to patients and families affected by these debilitating genetic disorders.
In particular, the approach to gene therapy in the case of Fragile X syndrome suggests innovative methods could alleviate symptoms by restoring the functionality of the FMR1 gene. Similarly, in Rett syndrome, where the MECP2 gene is mutated, gene therapy could provide a mechanism to introduce healthy copies or reactivate silenced pathways. Through further optimization and safety studies, these methods could pave the way forward for therapies that not only alleviate symptoms but fundamentally alter the disease landscape for those impacted by X-linked disorders, shifting the focus from management to potential cure.
Exploratory Findings in Chromosomal Research
The landscape of chromosomal research is continually evolving, driven by paradigm-shifting discoveries that enhance our understanding of genetic disorders. Studies by Jeannie T. Lee and her collaborators contribute significantly to this body of knowledge, particularly regarding the dynamics of X chromosome inactivation and its implications for human health. Their work is not only fundamental in answering core biological questions but also reveals pathways for therapeutic intervention that could profoundly impact those who suffer from genetic conditions like Fragile X and Rett syndromes. This is indicative of a larger trend within genetics, where basic research translates into potential clinical applications, bridging the gap between laboratory findings and real-world disease solutions.
Moreover, as the understanding of gene regulation deepens, the focus on chromosomal mechanisms highlights potential external factors that may influence gene expression, such as environmental triggers or epigenetic modifications. The advances in chromosomal research underscore the importance of interdisciplinary approaches, merging molecular biology, genetics, and even computational models to dissect genetic complexities. As researchers continue to explore these avenues, collaborative efforts will be essential in accelerating the breakthroughs needed to tackle genetic disorders head-on.
Challenges and Future Directions in Genetic Research
While the strides made in understanding X chromosome inactivation and its implications for genetic disorders are noteworthy, significant challenges remain in the field of genetic research. One major hurdle is the complexity of gene interactions and how mutations in key genes can lead to a myriad of clinical outcomes. For example, while the reactivation of inactivated genes may restore functionality to some extent, the precise mechanisms by which these therapies exert their effects are still not fully understood. This uncertainty complicates the development of targeted treatments, necessitating thorough research to elucidate these mechanisms before introducing them into clinical practice, particularly for conditions like Fragile X syndrome and Rett syndrome.
Looking to the future, it will be crucial for researchers to address these challenges head-on, fostering an environment of innovation and collaboration within the scientific community. The potential to utilize findings from chromosomal research to inform gene therapy strategies underscores the need for increased funding and support for sustained investigations into the genetic and molecular underpinnings of these disorders. As researchers deepen their understanding of XCI, Jell-O-like substances, and gene regulation, they will unlock promising therapies that could radically change the prognosis for individuals living with these genetic conditions.
The Intersection of X Chromosome Inactivation and Disease
Exploring the intersection of X chromosome inactivation and genetic diseases offers a unique perspective on the complexities involved in these conditions. X chromosome inactivation serves as a natural mechanism to balance gene dosage between sexes, but it also introduces challenges when considering genetic disorders linked to mutations in X-linked genes. Understanding how inactivated chromosomes can mask healthy gene functions while allowing mutations to express themselves has significant implications for therapeutic strategies. Jeannie T. Lee’s research aims to shed light on these connections, ultimately leading to insights that could revolutionize treatment pathways for various genetic disorders, including Fragile X syndrome and Rett syndrome.
As studies in this area advance, the potential to translate these findings into tangible therapies grows stronger. Recent discoveries indicate that the processes involved in XCI could be manipulated to unsilence mutated genes, offering a viable route for treatment. Furthermore, as researchers continue to investigate the cellular mechanisms underpinning X chromosome dynamics, the prospect of developing effective gene therapies becomes increasingly feasible. Breaking down the barriers between basic research and clinical application is paramount in addressing the needs of individuals affected by X-linked genetic disorders.
Innovative Strategies in Genetic Disorder Treatments
As the understanding of genetic disorders deepens, innovative strategies are emerging to target treatment at the root causes of conditions such as Fragile X syndrome and Rett syndrome. Rather than merely managing symptoms, recent research has begun to focus on reversing the biological underpinnings of these disorders through advanced gene therapy techniques. With a particular emphasis on X chromosome inactivation, scientists are exploring ways to reactivate healthy gene copies that are currently silenced due to their location on the inactivated X chromosome, raising hopes for effective new treatments.
The potential for these innovative strategies lies in their ability to provide long-term solutions rather than temporary fixes for genetic disorders. Jeannie T. Lee’s findings underscore the importance of these advances, as they illuminate how manipulating the physical and molecular environment of chromosomes can lead to therapeutic breakthroughs. With ongoing research in this field positioned to redefine how we approach the treatment of X-linked conditions, clinical trials may soon emerge that will test these innovative approaches, ultimately benefiting patients and their families.
Ethical Considerations in Genetic Research and Therapy
As the field of genetic research and therapy expands, ethical considerations surrounding these advancements become increasingly salient. The potential to manipulate gene expression, specifically through techniques that target X chromosome inactivation, raises important questions about the implications of such interventions. For conditions like Fragile X syndrome and Rett syndrome, the promise of effective treatments must be weighed against the ethical responsibility researchers have to consider the long-term effects on individuals and their families. The prospect of unsilencing mutated genes is exciting, yet it necessitates rigorous ethical evaluation to ensure that interventions are safe, equitable, and respectful of patient autonomy.
Moreover, as gene therapy advances move closer to clinical application, it is critical to engage with various stakeholders, including patients, ethicists, and legal experts, to navigate the complexities of these emerging therapies. Addressing issues such as informed consent, potential disproportionate effects on certain populations, and the implications of altering genetic material requires thoughtful discourse within the scientific community. By prioritizing ethical considerations in genetic research, the field can forge a responsible path forward that harmonizes innovation with respect for human dignity.
Frequently Asked Questions
What is X chromosome inactivation and how does it relate to genetic disorders like Fragile X syndrome and Rett syndrome?
X chromosome inactivation is a biological process that occurs in female mammals, where one of the two X chromosomes is inactivated to ensure equal gene dosage between males and females. This inactivation is crucial in understanding genetic disorders such as Fragile X syndrome and Rett syndrome, both of which arise from mutations on the X chromosome. By silencing one X chromosome, females can carry mutations without expressing the disorder, highlighting the significance of this process in chromosomal research.
How could findings from research on X chromosome inactivation lead to advancements in gene therapy?
Research on X chromosome inactivation, particularly the role of the Xist RNA molecule, may pave the way for innovative gene therapy approaches. By understanding how to manipulate the inactivation process, scientists aim to unsilence X-linked genes, providing potential treatments for genetic disorders like Fragile X syndrome and Rett syndrome. This advancement could allow healthy versions of genes to be expressed, presenting a significant breakthrough in therapeutic strategies.
What role does the ‘Jell-O-like substance’ play in X chromosome inactivation and its implications for chromosomal research?
The ‘Jell-O-like substance’ refers to a gelatinous material surrounding chromosomes that facilitates the process of X chromosome inactivation. This substance allows Xist RNA to infiltrate and modify its properties, ultimately leading to the silencing of one X chromosome. Understanding this mechanism is vital in chromosomal research, as it could provide insights into treating genetic disorders linked to X chromosome mutations, such as Fragile X syndrome and Rett syndrome.
Can X chromosome inactivation strategies potentially cure Fragile X syndrome and Rett syndrome?
Yes, by unlocking the inactivated X chromosome, researchers believe they can restore the function of mutated genes associated with Fragile X syndrome and Rett syndrome. This could enable the expression of healthy genes that were previously silenced, providing a promising therapeutic avenue for those affected by these genetic disorders. The ongoing optimization of these techniques aims to maximize the clinical viability of such treatments.
What are the implications of X chromosome inactivation on the understanding of genetic disorders?
The study of X chromosome inactivation has significant implications for understanding genetic disorders, particularly those caused by mutations on the X chromosome. It reveals how certain diseases, like Fragile X syndrome and Rett syndrome, are expressed depending on gene activity and dosage. Insights gained from this research may inform the development of targeted therapies that selectively unsilence beneficial genes, potentially transforming treatment modalities for affected individuals.
How has research on X chromosome inactivation evolved over recent decades?
Research on X chromosome inactivation has evolved significantly, shifting from fundamental questions of how this process occurs to exploring its therapeutic implications. The work by Jeannie T. Lee and her team illustrates this evolution, as they have discovered mechanisms that could lead to innovative treatments for genetic disorders such as Fragile X syndrome and Rett syndrome, showcasing how basic research can lead to impactful clinical applications.
| Key Points | Details |
|---|---|
| X Chromosome Challenge | Females have two X chromosomes while males have one, requiring one to be inactivated. |
| Role of Xist | A gene on the X chromosome produces the Xist RNA which plays a critical role in X inactivation. |
| Biophysical Properties of Chromosomes | Xist alters the gelatinous substance (referred to as Jell-O) around the X chromosome. |
| Therapeutic Potential | Uncovering mechanisms of X inactivation may lead to treatments for Fragile X and Rett syndromes. |
| Minimal Side Effects | Restoring function of mutated genes without affecting healthy genes could lead to safer treatment options. |
| Future Research | Ongoing studies will focus on optimizing treatment approaches and ensuring safety before clinical trials. |
Summary
X chromosome inactivation is a crucial biological mechanism that allows females, who have two X chromosomes, to silence one copy to maintain gene dosage balance. Recent research led by Jeannie T. Lee has unveiled significant insights into how this process occurs, highlighting the role of the Xist RNA and the surrounding biophysical environment. This understanding opens possibilities for innovative therapies targeting diseases related to mutations on the X chromosome, such as Fragile X syndrome and Rett syndrome. As the research progresses, the hope for developing effective treatments with minimal side effects continues to grow.